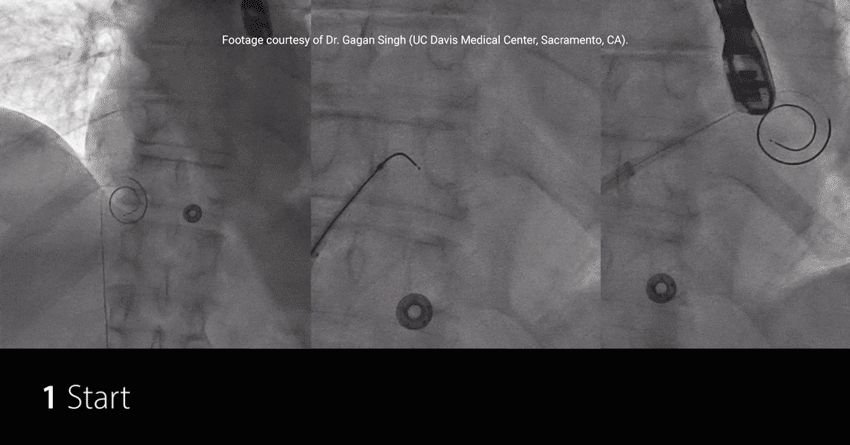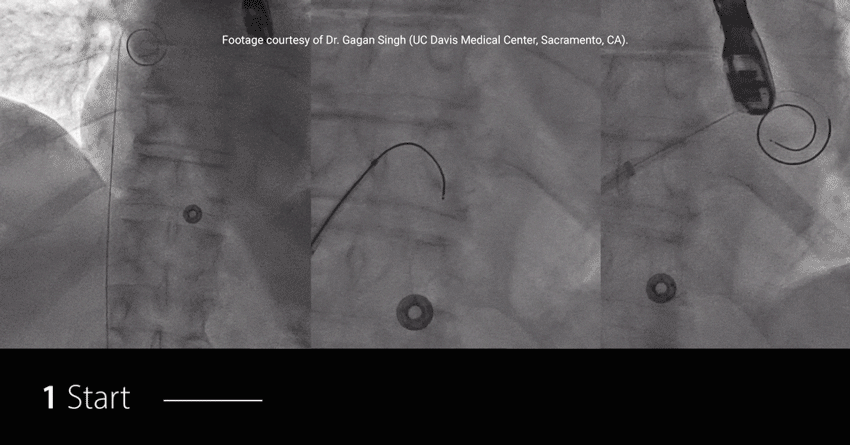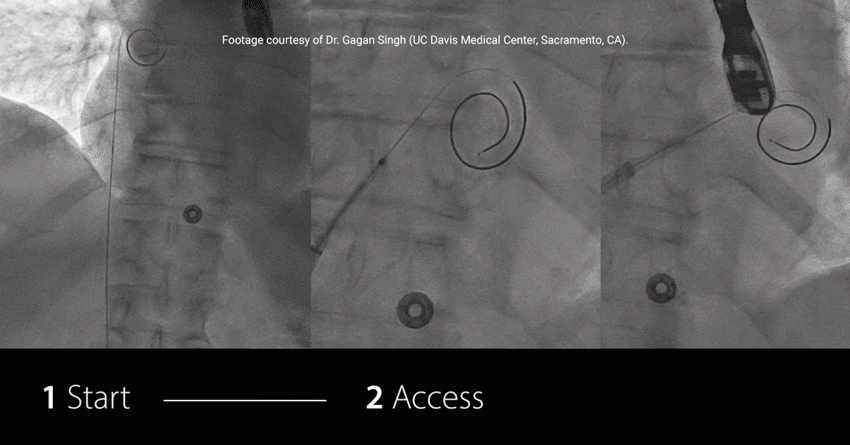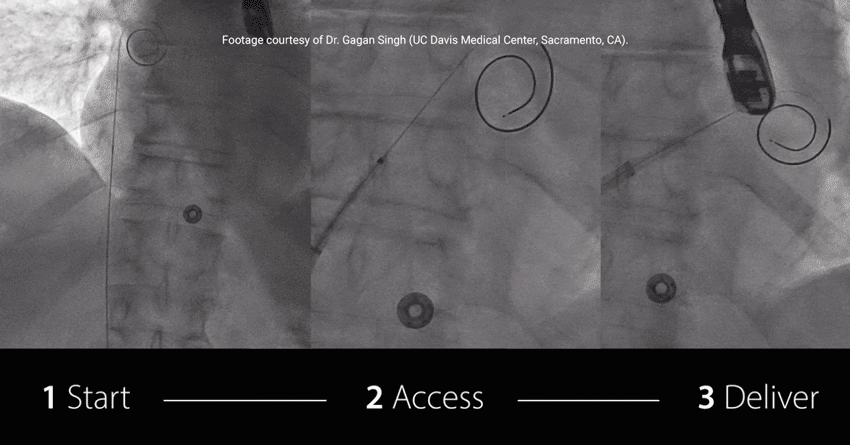
Initial Clinical Experience with VersaCross® Transseptal System for
Transcatheter Mitral Valve Repair, study led by Dr. Anita Asgar
|
Study highlights using RF wire:
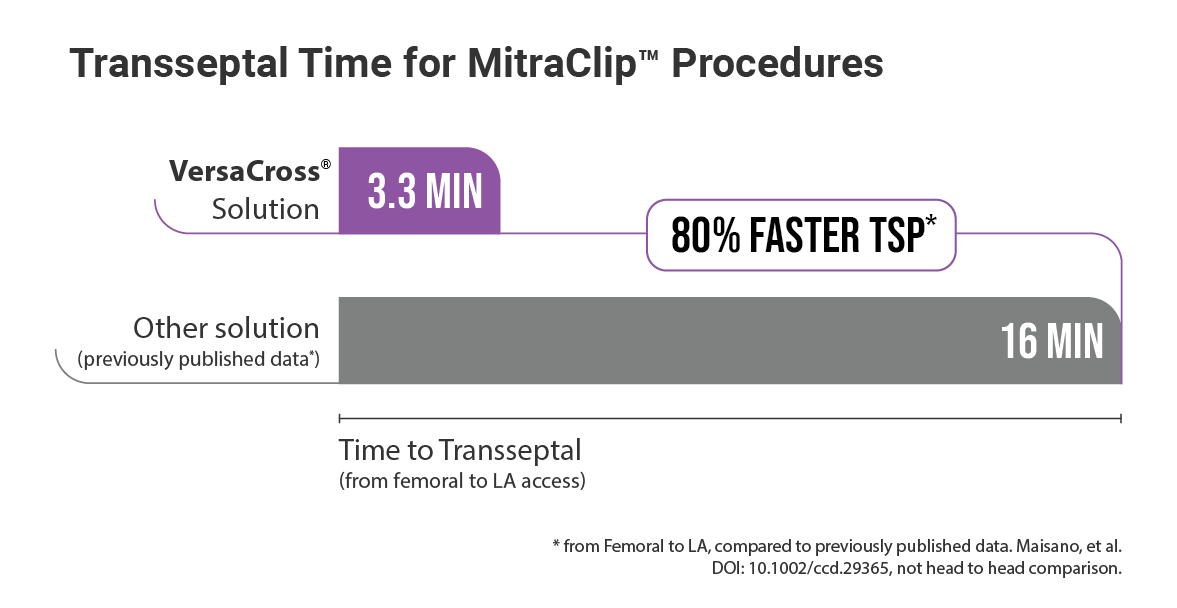
EFFICIENT: Achieved TSP and MitraClip™ Guide delivery in under 7.5 min†
EXCHANGELESS: Reduced number of wire exchanges
EFFORTLESS: Repositioned on the fossa with no rewiring
|
|
Dr. Anita Asgar presents on new techniques for streamlined transseptal
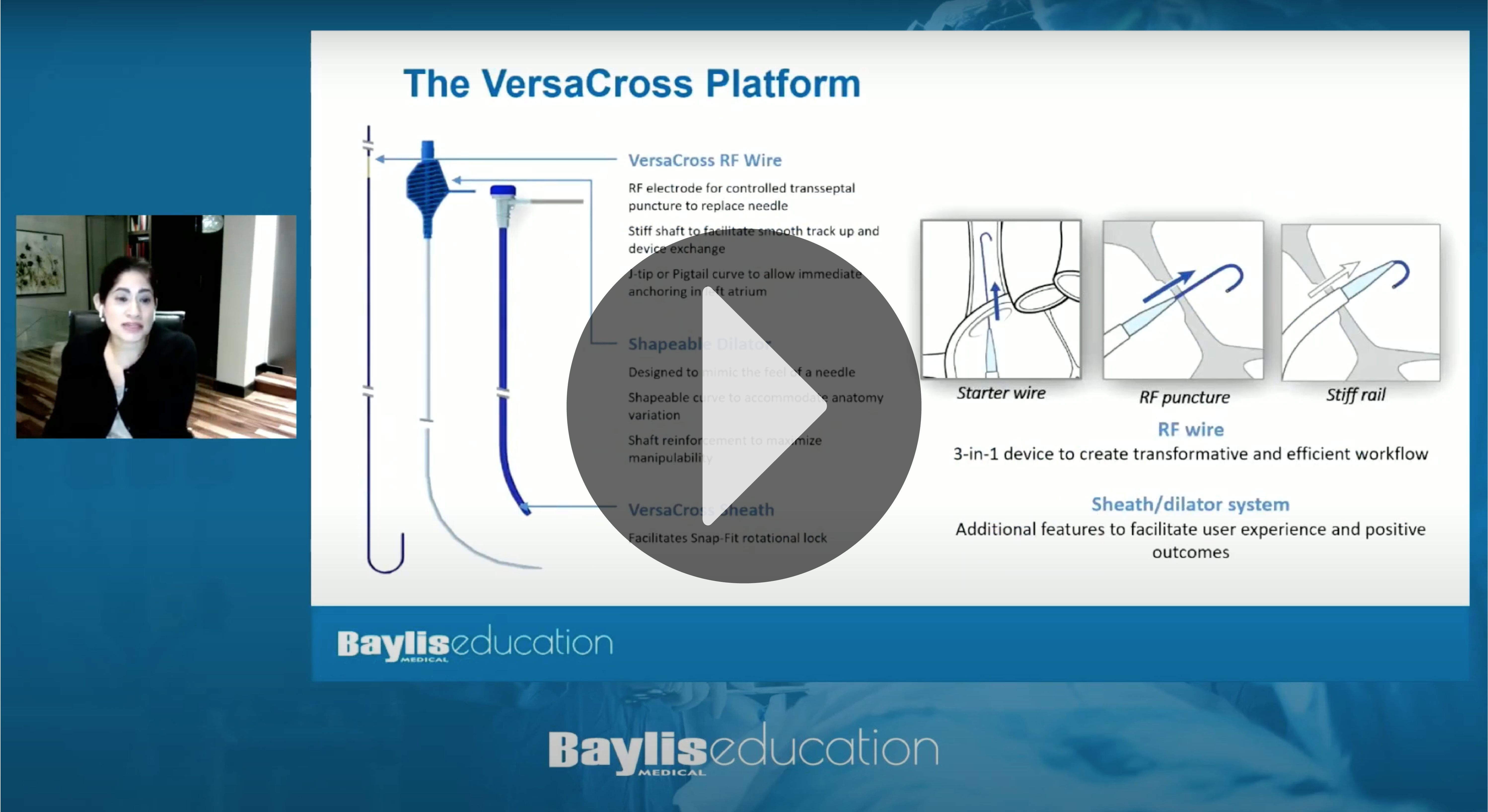 |

Single Solution.
No Exchanges.™
World’s first exchangeless solution for access-to-delivery of left heart therapy devices.
|
|
*Not based on head-to-head comparison, Maisano, et al. DOI: 10.1002/ccd.29365
† Using the VersaCross® RF Transseptal Solution from femoral access; based on 3.3 min for TSP and 3.8 min for subsequent MitraClip guide exchange.
VersaCross® RF Transseptal Solution is intended for left heart access. The VersaCross® RF Wire is indicated for creation of an atrial septal defect in the heart. VersaCross® Transseptal Sheath is indicated for introducing cardiovascular catheters and guidewires to the left atrium.
PRM-00667 EN J-1,2,3 V-2 © Copyright Baylis Medical Company Inc., 2020-2021. Baylis Medical Company Inc. reserves the right to change specifications or to incorporate design changes without notice and without incurring any obligation relating to equipment previously manufactured or delivered. VersaCross, the VersaCross logo, 'Single Solution. No Exchanges.", Baylis Medical and the Baylis Medical logo are trademarks and/or registered trademarks of Baylis Medical Company Inc. in the USA and/or other countries. Other trademarks are property of their respective owners. Patents Pending and/or issued. CAUTION: Federal Law (USA) restricts the use of these devices to or by the order of a physician. Before use, consult product labels and Instructions for Use for Indications for Use, Contraindications, Warnings, Precautions, Adverse Events and Directions for Use. Products shown may not be approved in all jurisdictions.
Thank you for subscription!