Labelling Transition to GS1 for Unique Device Identification (UDI) Compliance
What is a Unique Device Identifier (UDI)?
A UDI is a numeric or alphanumeric identification code assigned to a medical device that uniquely identifies the device throughout its distribution and use.
The FDA’s UDI Final Rule (September 2013) requires a UDI on every device label and package for medical devices commercially distributed in the United States. The aim of the UDI system is to make it easier to identify medical devices, which will facilitate more accurate reporting, review and analysis of device information.
For more information on establishment of the UDI system by the FDA visit: FDA Unique Device Identification - UDI
What is GS1?
GS1 is a neutral, not-for-profit, global organization that develops and maintains the most widely used supply chain standards system in the world. GS1 provides tools and standards for identification and publication of product data across all industries and geographies.
For more information on GS1 visit: Welcome to GS1 Canada
How does GS1 relate to UDI?
GS1 is an FDA-accredited issuing agency for assignment of UDIs. Baylis Medical has adopted use of GS1 barcodes as part of its UDI compliance program.
What information is embedded in the barcode on Baylis Medical devices?
UDI = device identifier (DI) + production identifier (PI)
Under the GS1 system, the fixed (DI) portion of the UDI is called a Global Trade Items Number (GTIN) and the variable (PI) portion of the UDI may include various types of production information.
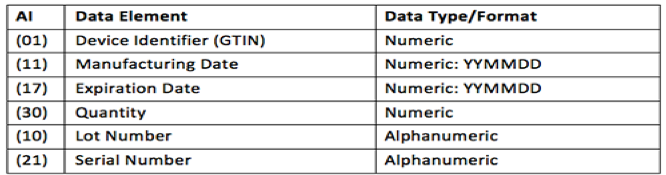
The Application Identifier (AI) appears in the human readable portion of the barcode to identify the data elements in the barcode. The table below shows the data elements that may be included in the barcode (i.e. each AI used, its meaning and the corresponding data format).
What is the timeline for implementing this new labelling?
The majority of Baylis Medical’s product labels have been updated to include the new GTIN identifier. Over the next year, you may see both HIBC-labeled and GTIN-labeled inventory.
Do I need to return inventory that doesn’t have the new GTIN labeling?
No. There is no difference between products labeled with HIBC barcodes and GS1 barcodes and as a result, there is no need to return the HIBC-labeled products.
Do I need to use GTINs to order Baylis Medical products? Will the invoice and packing slips change?
No. You may continue to order products using model/catalogue numbers. The invoice and packing slips will remain the same.
If you have any questions about Baylis Medical’s labelling transition to GS1 for UDI Compliance please contact us: For more information